IMPORTANT SAFETY INFORMATION FOR TRIPTODUR
INDICATION
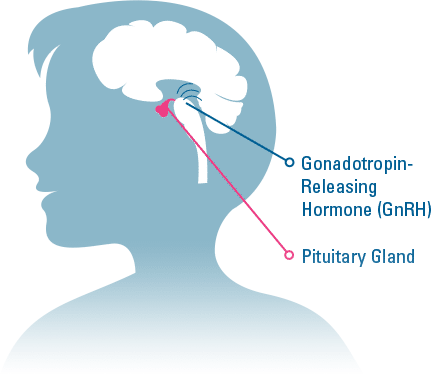
TRIPTODUR is indicated for the treatment of pediatric patients 2 years of age and older with central precocious puberty (CPP).
IMPORTANT SAFETY INFORMATION
Do not use TRIPTODUR in:
- Those allergic to gonadotropin releasing hormone (GnRH), GnRH agonist medicines, or any ingredients in TRIPTODUR.
- children under 2 years of age
- women who are or may become pregnant
Tell your child’s healthcare provider if any of the above conditions apply to your child.
It is important to stick to the dosing schedule (one injection every 24 weeks) in order for the drug to work. Do not miss or delay a scheduled dose.
Some people taking gonadotropin releasing hormone (GnRH) agonists like TRIPTODUR have had new or worsened mental (psychiatric) problems. Mental (psychiatric) problems may include emotional symptoms such as crying, irritability, restlessness (impatience), anger, or acting aggressive. Call your child’s doctor right away if your child has any new or worsening emotional symptoms while taking TRIPTODUR.
Some people taking GnRH agonists like TRIPTODUR have had seizures. The risk of seizures may be higher in people who have a history of seizures, have a history of epilepsy, have a history of brain or brain vessel (cerebrovascular) problems or tumors, are taking a medicine that has been connected with seizures such as bupropion or selective serotonin reuptake inhibitors (SSRIs). Seizures have also happened in people who have not had any of these problems. Call your child’s doctor right away if your child has a seizure while taking TRIPTODUR.
Some people taking triptorelin, the active ingredient in TRIPTODUR, have had serious allergic reactions. Call your child’s doctor or get emergency medical help right away if your child gets any of the following symptoms of a serious allergic reaction: skin rashes, redness, or swelling, severe itching, hives, trouble breathing or swallowing, fast heartbeat, sweating, throat tightness, hoarseness, swelling of face, mouth, and tongue, dizziness or fainting.
The most common side effects of TRIPTODUR include injection site reactions, menstrual (vaginal) bleeding, hot flush, headache, cough, and infections (bronchitis, gastroenteritis, influenza, nasopharyngitis, otitis externa, pharyngitis, sinusitis, and upper respiratory tract infection). These are not all the possible side effects of TRIPTODUR. Tell your child’s healthcare provider if they have any side effect that bothers them or that does not go away.
In the first few weeks after your child receives their first TRIPTODUR injection or after additional injections, TRIPTODUR can cause a brief increase in some hormones. During this time you may notice more signs of puberty in your child, including vaginal bleeding. Call your child’s doctor if signs of puberty continue after 2 months of receiving TRIPTODUR.
Reports of pseudotumor cerebri (idiopathic intracranial hypertension) have been observed in pediatric patients receiving GnRH agonists, including triptorelin. Patients and caregivers should contact their healthcare provider if the patient develops any of following symptoms of pseudotumor cerebri, including headache, and vision issues such as blurred vision, double vision, loss of vision, pain behind the eye or pain with eye movement, ringing in the ears, dizziness, and nausea.
These are not all the possible side effects of TRIPTODUR. Call your doctor for medical advice about side effects.
To report SUSPECTED ADVERSE REACTIONS, contact Azurity Pharmaceuticals, Inc. at 1-800-461-7449, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
The Important Safety Information does not include all the information needed to use TRIPTODUR safely and effectively. For additional safety information, please consult the full Prescribing Information for PDF Document: TRIPTODUR (File Size: 153 KB).